In this post Standard Operating Procedure of Request of Additional Packing Material in pharmaceuticals is describe.
1.0 OBJECTIVE:
To lay down a procedure for request of additional packing material.
2.0 SCOPE:
This procedure is applicable for request of additional packing material in production department.
3.0 RESPONSIBILITY:
Officer, Executive Production Department and Quality Assurance – for Execution Head production- shall ensure compliance and implementation of the SOP.
4.0 DEFINITION(S):
NA
5.0 PROCEDURE:
5.1 When the consumption of packing material for a batch is more than the standard quantity, then raise additional packing material requisition as per the standard format in BPR
5.2 Authorize the additional packing material requisition by the head QA and head production, Generate the additional material request in the ERP as per given procedure –
5.2.1 Open the WORK ORDER DEVIATED ISSUE REQUEST option in the ERP.
5.2.2 Click on Add New option as shows in the screen -
5.2.3 On the new screen Enter the WORK ORDER number and OPERATION code as 1 and then go for the WORKORDER DETAILS column as shown in the screen -
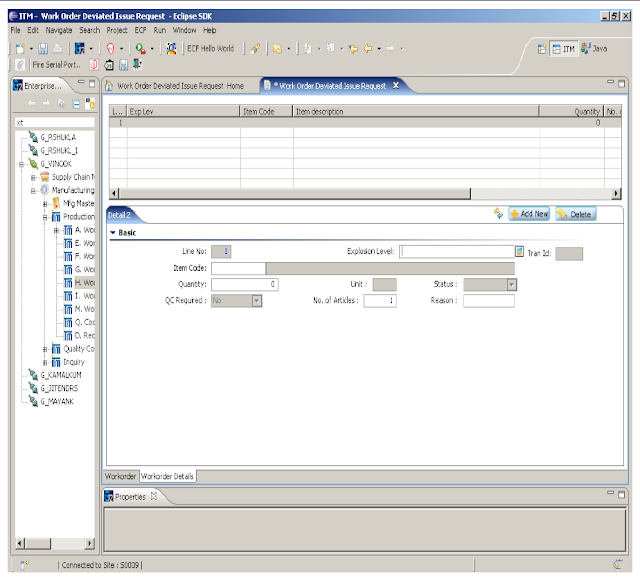
5.2.4 After entering on the WORKORDER DETAILS new screen opens as -
5.2.5 Enter the details in the EXPLOSION LEVEL, QUANTITY and REASON in the respective field with the help of popup. The Quantity should be same as in the BPR copy for the additional material request. Add more then one material request by ADD NEW option if required. Save the request.
5.3 Send the additional packing material request to the store .
5.4 Ensure that the quantity issued is as per the requisition.
5.5 Transfer the dispensed additional packing material to the primary/secondary packing material area.
6.0 ABBREVIATION(S):
BPR: Batch Packing Record. QA : Quality Assurance
SOP: Standard Operating Procedure ERP: Enterprises Resource Planning
7.0 RERERENCE(S):
NA
8.0 ANNEXURE(S):
Nil
9.0 DISTRIBUTION:
9.1 Master Copy: Quality Assurance
9.2 Controlled Copy (s): Production department, Quality Assurance.
9.3 Reference Copy (s): Production department (2 copy)





.webp)







.webp)
