In this post Standard Operating Procedure Cleaning and Operation of Bin Blender (Capacity 4.5 Liters) in Pharmaceuticals is describe.
1.0 OBJECTIVE:
1.1 To lay down a procedure for Cleaning and Operation of Bin Blender (Capacity 4.50 liters).
2.0 SCOPE:
2.1 The procedure is applicable to the Cleaning and Operation of Bin Blender (Capacity 4.50 liters) in production department.
3.0 RESPONSIBILITY:
3.1 Technical Associate : Cleaning and Operation
3.2 Officer and Executive : Supervision
3.3 Head Production : SOP compliance
3.4 IPQA Person : Line Clearance
4.0 DEFINITION(S):
4.1 NA
5.0 PROCEDURE:
5.1 “TYPE A” CLEANING:
Change over from one batch to next batch of the same product, same potency and have similar product with ascending potency.
5.1.1 Ensure the door is closed properly.
5.1.2 Affix dully filled “TO BE CLEANED” status label on equipment with date and signature of the Production Officer as per SOP No.: …………….
5.1.3 Clean the blender and bin with dry lint free cloth.
5.1.4 Enter the cleaning start time of equipment in equipment usage log sheet as per SOP No.: ………….
5.1.5 Replace the “TO BE CLEANED “status label with “CLEAED” status label with date and signature of the Production/QA Officer.
5.1.6 Record the cleaning observations in the equipment usage log sheet as per SOP No. …………
5.2 “TYPE B” CLEANING:
This is a cleaning procedure for Change over of product with different actives / colour / descending potency or after maintenance of contact parts.
5.2.1 Affix dully filled “TO BE CLEANED” status label on equipment with date and signature of the Production Officer as per SOP No.: …………….
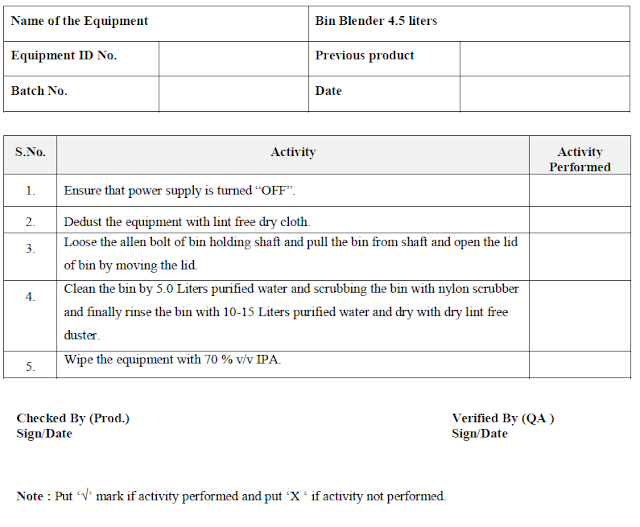
5.2.2 Ensure that power supply is turned “OFF”.
5.2.3 Dedust the equipment with lint free dry cloth.
5.2.4 Loose the allen bolt of bin holding shaft and pull the bin from shaft and open the lid of bin by moving the lid.
5.2.5 Clean the bin by 5.0 Liters purified water and scrubbing the bin with nylon scrubber and finally rinse the bin with 10-15 Liters purified water and dry with dry lint free duster.
5.2.6 Wipe the equipment with 70 % v/v IPA.
5.2.7 Affix dully filled and signed ‘CLEANED’ label on the equipment.
5.2.8 Record all the observations in the Equipment usage log sheet as per SOP No. …………
5.2.9 If cleaned equipment is not used for 72 hours, vipe all the parts of equipment with 70% v/v IPA solution before use. And should be a Counter sign on previous “CLEANED” label by production & QA officer with date as per SOP No.: ……………
5.2.10 Record the 70 % v/v IPA cleaning time of equipment in equipment usage logbook as per SOP No.: …………..
5.2.11 Record the activity in cleaning checklist Annexure I
5.3 Frequency
5.3.1 Type ‘A’ cleaning is applicable after completion of every batch of same product.
5.3.2 Type ‘B’ cleaning is applicable in case of product change over or same product is run for a week or which ever is earlier.
5.4 MACHINE OPERATION:
5.4.1 Ensure ‘CLEANED’ label duly filled and signed is affixed on the equipment. Ensure cleanliness of equipment and area. Remove the ‘CLEANED’ label and affix it in the respective BMR. Affix UNDER PROCESS’ label duly filled and signed on the equipment and records all the observations in the Equipment Usage Log Sheet as per SOP No. …………..
5.4.2 Load the powder in the bin of blender.
5.4.3 Turn ‘ON’ the control ‘ON/OFF’ key.
5.4.4 Set the RPM by RPM knob as per specified in BMR.
5.4.5 Set the time in the timer as per BMR.
5.4.6 Press ON button
5.4.7 To stop the blender in emergency.
5.4.8 After completion of blending unload the bin and kept the blend in SS bin lined with double polybag and store in blend quarantine area and enter in Inward outward register as per SOP No………..
5.4.9 Affix ‘TO BE CLEANED’ label duly filled and signed on the bin blender.
5.4.10 Record all the observations in equipment Usage Log Sheet as per SOP No………….
6.0 ABBREVIATION (S):
6.1 IPA : Iso propyl alcohol
6.2 SOP : Standard Operating Procedure.
6.3 No. : Number
6.4 v/v : Volume/Volume
6.5 BMR : Batch Manufacturing Record
6.6 QA : Quality Assurance
6.7 SOP : Standard Operating Procedure.
7.0 REFERENCE (S):
7.1 SOP No. PG/178, Making entries in equipment usage and cleaning log sheet.
7.2 SOP No. PG/175, Cleaning of production area
7.3 SOP No. QA/044, Status Labeling
8.0 ANNEXURE (S):
9.0 DISTRIBUTION:
9.1 Master Copy (S) : Quality Assurance
9.2 Controlled Copy (S): Production department (02) , Quality Assurance (01)
9.3 Reference Copy (S): Production department (01)
ANNEXURE I
CLEANING CHECKLIST OF BIN BLENDER 4.5 liters

.webp)



.webp)







%20Web%20of%20pharma%20.webp)
